PSAP Rabbit mAb [Lr76]Cat NO.: A20865
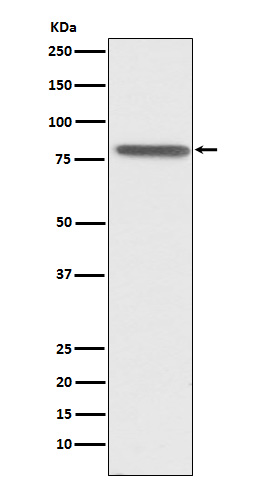
Western blot(SDS PAGE) analysis of extracts from HepG2 cell lysate.Using PSAP Rabbit mAb [Lr76]at dilution of 1:1000 incubated at 4℃ over night.
Product information
Protein names :GLBA; SAP1; Saposins; Dispersin;
UniProtID :P07602
MASS(da) :58,113
MW(kDa) :78kDa
Form :Liquid
Purification :Affinity-chromatography
Host :Rabbit
Isotype : IgG
sensitivity :Endogenous
Reactivity :Human
- ApplicationDilution
- 免疫印迹(WB)1:1000-2000
- 免疫组化(IHC)1:100
- The optimal dilutions should be determined by the end user
Specificity :Antibody is produced by immunizing animals with A synthesized peptide derived from human PSAP
Storage :Antibody store in 10 mM PBS, 0.5mg/ml BSA, 50% glycerol. Shipped at 4°C. Store at-20°C or -80°C. Products are valid for one natural year of receipt.Avoid repeated freeze / thaw cycles.
WB Positive detected :HepG2 cell lysate.
Function : Saposin-A and saposin-C stimulate the hydrolysis of glucosylceramide by beta-glucosylceramidase (EC 3.2.1.45) and galactosylceramide by beta-galactosylceramidase (EC 3.2.1.46). Saposin-C apparently acts by combining with the enzyme and acidic lipid to form an activated complex, rather than by solubilizing the substrate., Saposin-B stimulates the hydrolysis of galacto-cerebroside sulfate by arylsulfatase A (EC 3.1.6.8), GM1 gangliosides by beta-galactosidase (EC 3.2.1.23) and globotriaosylceramide by alpha-galactosidase A (EC 3.2.1.22). Saposin-B forms a solubilizing complex with the substrates of the sphingolipid hydrolases., Saposin-D is a specific sphingomyelin phosphodiesterase activator (EC 3.1.4.12)., [Prosaposin]: Behaves as a myelinotrophic and neurotrophic factor, these effects are mediated by its G-protein-coupled receptors, GPR37 and GPR37L1, undergoing ligand-mediated internalization followed by ERK phosphorylation signaling.., Saposins are specific low-molecular mass non-enzymic proteins, they participate in the lysosomal degradation of sphingolipids, which takes place by the sequential action of specific hydrolases.
Subcellular locationi :Lysosome.,[Prosaposin]: Secreted.
IMPORTANT: For western blots, incubate membrane with diluted primary antibody in 1% w/v BSA, 1X TBST at 4°C overnight.